Abstract
Introduction: In the primary analysis of the phase 3 MAIA trial (NCT02252172), daratumumab, lenalidomide and dexamethasone (D-Rd) improved progression-free survival (PFS) in transplant-ineligible (TIE) patients (pts) with newly diagnosed multiple myeloma (NDMM) vs Rd. With longer follow-up (median: 56.2 months [mo]), D-Rd continued to demonstrate PFS benefit, conferred an overall survival benefit, induced deeper responses, and was associated with clinically meaningful improvements in patient-reported outcomes (PROs) vs Rd. The median age of these pts was 73 years with 43.6% of pts aged ≥75 years. Consistent with the overall population, in a frailty subgroup analysis (median age: 77 years), D-Rd also demonstrated deeper responses and PFS benefit vs Rd (not reached [NR] vs 30.4 mo; HR, 0.62; P = 0.003) in frail pts at a median follow-up of 36.4 mo. Although, D-Rd improved clinical outcomes in these elderly frail pts, little is known about health-related quality of life (HRQoL) outcomes. In frail pts, improving HRQoL and minimizing the risk of toxicity is paramount. Here, we assess PROs in the frail pts in the MAIA trial.
Methods: Pts were randomized 1:1 to D-Rd or Rd until disease progression (PD) or unacceptable toxicity. Frailty assessment was performed on all randomized pts using age, Charlson Comorbidity Index, and baseline (BL) Eastern Cooperative Oncology Group performance status score. Based on this assessment, pts were categorized into frail and non-frail subgroups. PROs were assessed using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30-item (EORTC QLQ-C30). Questionnaires were completed at BL, on day 1 of cycles (Cyc) 3, 6, 9, and 12 for year 1, and every 6 mo thereafter until PD. Analyses were conducted on all pts with a BL and ≥1 post-BL PRO assessment. Meaningful thresholds for improvement and worsening were defined a priori based on published literature (≥10-point change). Treatment effect was assessed by a mixed-effects model for repeated measures. Time to first worsening was estimated using the Kaplan Meier method and Cox proportional hazards regression was used to compute hazard ratio (HR) and associated 95% confidence interval (CI) where worsening was defined using a distribution-based method.
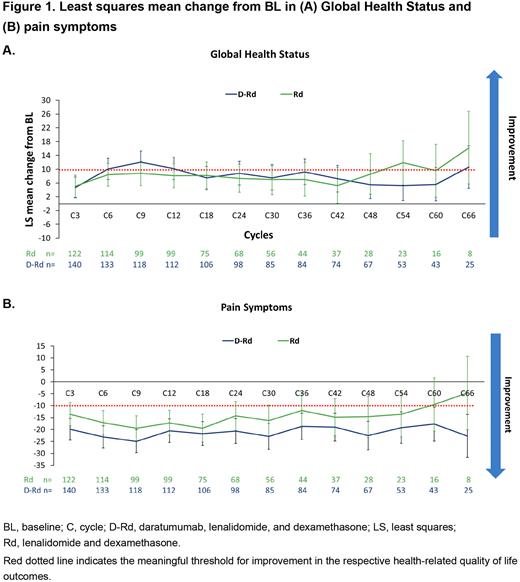
Results: In the MAIA trial, 737 pts were randomized (D-Rd, n=368; Rd, n=369). Of these, 341 pts were classified as frail (D-Rd, n=172; Rd, n=169,). At a median follow-up of 64.5 mo, frail pts treated with D-Rd reported greater improvements from BL vs Rd in EORTC QLQ-C30 Global Health Status (GHS) scores (Figure 1A) and physical functioning (LS mean change from BL for D-Rd at Cyc 12: 12.7 [9.0-16.3] vs Rd, 9.8 [5.9-13.7]) at several time points, with 39% of pts treated with D-Rd staying on treatment with PRO assessment at Cyc 48 vs 17% in the Rd group. Additionally, pts treated with D-Rd showed notably large reductions (≥20-point change) in pain symptoms over time and larger than that seen in pts treated with Rd (Figure 1B). No meaningful changes from BL were observed in fatigue symptoms for either treatment group. Results for emotional functioning, social functioning, and nausea and vomiting also numerically favored D-Rd at several time points. The median time to first improvement was numerically shorter with D-Rd vs Rd for GHS (D-Rd: 2.38 mo vs Rd: 4.67 mo), physical functioning (2.60 mo vs 4.65 mo), pain (2.07 mo vs 2.83 mo), and fatigue symptoms (2.04 mo vs 3.09 mo). The median time to first worsening was longest for physical functioning (D-Rd: 68.14 mo vs Rd: 39.62 mo; HR [95%CI] 0.66 [0.45-0.95]; P=0.023) and pain symptoms (D-Rd: NR vs Rd: 60.48 mo; 0.66 [0.44-1.00]; P=0.045). The median time to first worsening was longer with D-Rd vs Rd for GHS (D-Rd: 29.31 mo vs Rd: 21.62 mo; HR 0.91 [0.65-1.26]). The median time to first worsening for the other functional (emotional, social, role) and symptom (nausea and vomiting) scales numerically favored D-Rd vs Rd, except cognitive functioning and fatigue.
Conclusions: D-Rd demonstrates clinical benefit in all pts, including frail pts. In addition, frail pts treated with D-Rd reported sustained improvements in global health (overall HRQoL) and physical functioning, with notable reduction in pain through the duration of therapy. Additionally, higher percentage of frail pts continued on D-Rd vs Rd. D-Rd regimen is not only clinically effective but also results in a sustained improvement in HRQoL for TIE frail pts with NDMM.
Disclosures
Perrot:Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Plesner:Janssen, Genmab, Celgene, Takeda, Oncopeptides, Genentech, AbbVie, Roche, Bristol Myers Squibb: Research Funding; Janssen, Celgene, Takeda, Oncopeptides, Genentech, CSL Behring, AbbVie: Membership on an entity's Board of Directors or advisory committees. Usmani:Abbvie, Amgen, BMS, Celgene, EdoPharma, Genentech, Gilead, GSK, Janssen,Oncopeptides, Sanofi, Seattle Genetics, SecuraBio, SkylineDX, Takeda, TeneoBio: Consultancy; Amgen, BMS, Janssen, Sanofi: Speakers Bureau; Amgen, Array Biopharma, BMS, Celgene, GSK, Janssen, Merck, Pharmacyclics, Sanofi, Seattle Genetics, SkylineDX, Takeda: Research Funding. Kumar:AbbVie,: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen,: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda,: Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive,: Membership on an entity's Board of Directors or advisory committees, Research Funding; KITE,: Research Funding; MedImmune/Astra Zeneca,: Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck,: Research Funding; Novartis,: Research Funding; Roche: Research Funding; Sanofi: Research Funding; Oncopeptides: Other: Independent review committee. Bahlis:AbbVie: Consultancy, Honoraria; Takeda: Consultancy; Amgen: Consultancy, Honoraria; Genentech: Consultancy; Celgene: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Sanofi: Consultancy, Honoraria; GSK: Consultancy, Other; Forus: Consultancy, Honoraria; Karyopharm Therapeutics: Consultancy, Honoraria. Hulin:Sanofi: Honoraria; Takeda: Honoraria; GSK: Honoraria; BMS: Honoraria; Janssen: Honoraria; Amgen: Honoraria. Orlowski:Asylia Therapeutics, Inc.: Current equity holder in private company; Abbvie, BioTheryX, Inc., Bristol-Myers Squibb, Janssen Biotech, Karyopharm Therapeutics, Inc., Meridian Therapeutics, Monte Rosa Therapeutics, Neoleukin Corporation, Oncopeptides AB, Regeneron Pharmaceuticals, Inc., Sanofi-Aventis, and Takeda Pharmaceutic: Honoraria, Membership on an entity's Board of Directors or advisory committees; CARsgen Therapeutics, Celgene/Bristol Myers Squibb, Exelixis, Janssen Biotech, Sanofi-Aventis, Takeda Pharmaceuticals North America, Inc.: Research Funding; Asylia Therapeutics, Inc., BioTheryX, Inc., Heidelberg Pharma, Inc.: Research Funding. Mollee:Janssen, Pfizer: Research Funding; Amgen, BMS, Caelum, EUSA Siltuximab, Janssen, Pfizer, SkylineDx, Takeda: Membership on an entity's Board of Directors or advisory committees. Roussel:GSK, Takeda, Janssen: Honoraria. Jaccard:sanofi: Research Funding; Amgen: Honoraria; Pfizer: Honoraria; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding. Delforge:Janssen: Honoraria, Speakers Bureau; BMS: Honoraria; Amgen: Honoraria; GSK: Honoraria. Karlin:Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Financial Support travel & scientific meetings; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Financial Support travel & scientific meetings; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene-BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees. Arnulf:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Pei:Janssen: Current Employment, Current equity holder in publicly-traded company. Gupta:Janssen Scientific Affairs, LLC: Current Employment, Current holder of stock options in a privately-held company. Kaila:Janssen Scientific Affairs, LLC: Current Employment. Matt:Janssen: Current Employment. Gries:Janssen Pharmaceutical: Current Employment, Current holder of stock options in a privately-held company. Carson:Janssen: Current Employment. Borgsten:Janssen: Current Employment. Weisel:Stemline: Honoraria; Roche: Consultancy, Honoraria; Oncopeptides: Consultancy, Honoraria; Karyopharm: Consultancy, Honoraria; GSK: Consultancy, Honoraria; BeiGene: Consultancy, Honoraria; AstraZeneca: Honoraria; Adaptive Biotech: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria; BMS/Celgene: Consultancy, Honoraria, Research Funding; Novartis: Honoraria; Amgen: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal